Write out the skeletal equation.
\(ZnS_{(s)} + O_{2(g)} \to ZnO_{(s)} + SO_{2(g)}\)
Apply your Knowledge
Learn the theory at your own pace, try examples and test your new abilities with the quiz.
(Click the banner to open.)
Working with Chemical Equations Theory
Terminology:
Click/tap to view definitions

We Know:
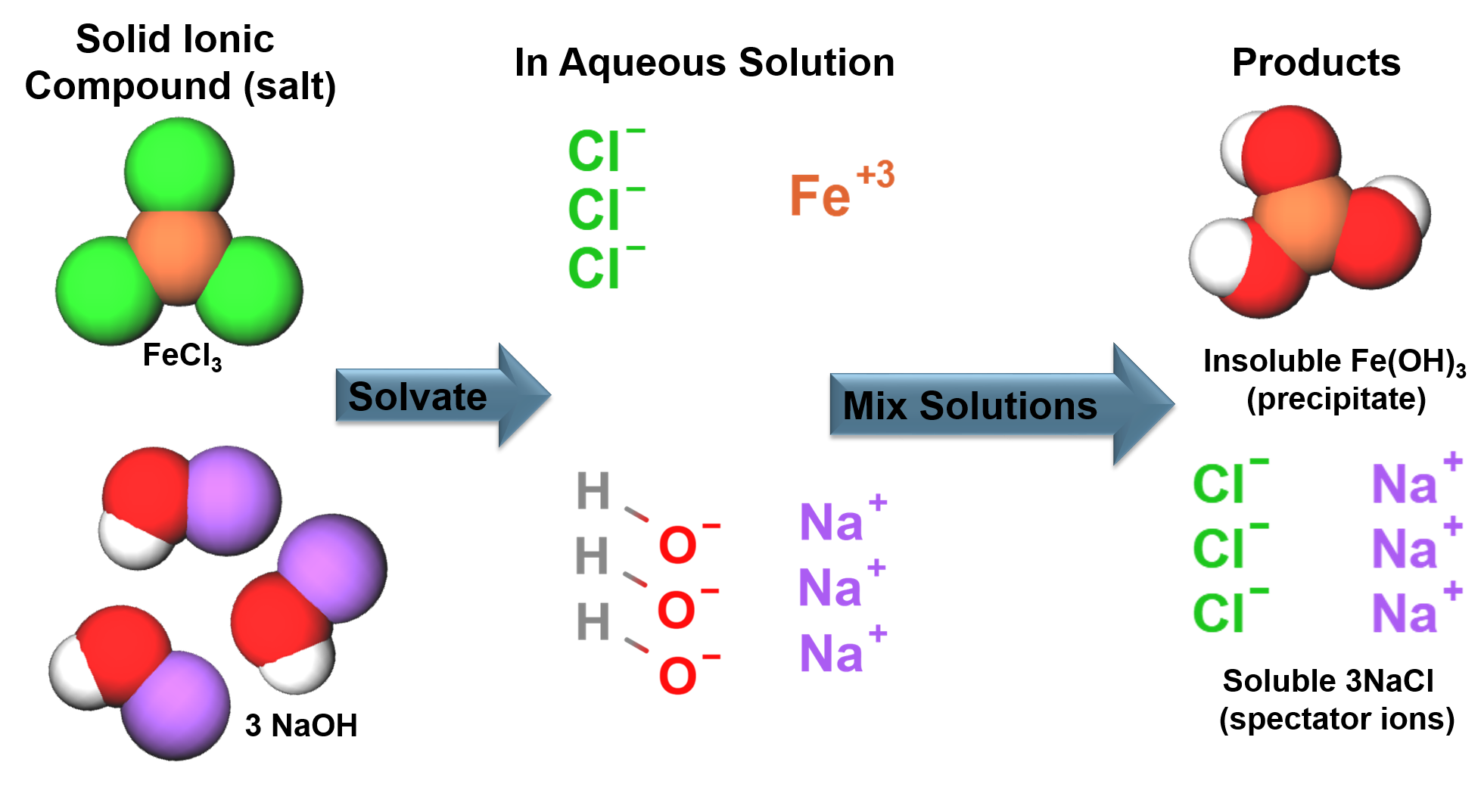
Elements combine in different ways to form substances.
All samples of a pure substance contain the same proportion of elements by mass.
Law of Conservation of Mass:
In a chemical reaction atoms are rearranged through the making and breaking chemical bonds, atoms are not altered or destroyed.
In a chemical reaction the mass of the products equals the mass of the reactants.
Law of Conservation of Mass: In a chemical reaction mass is neither created or destroyed.
We Know:
All atoms in the reactants must be accounted for in the products! (Conservation of mass).
Chemical reactions are written as reaction equations.
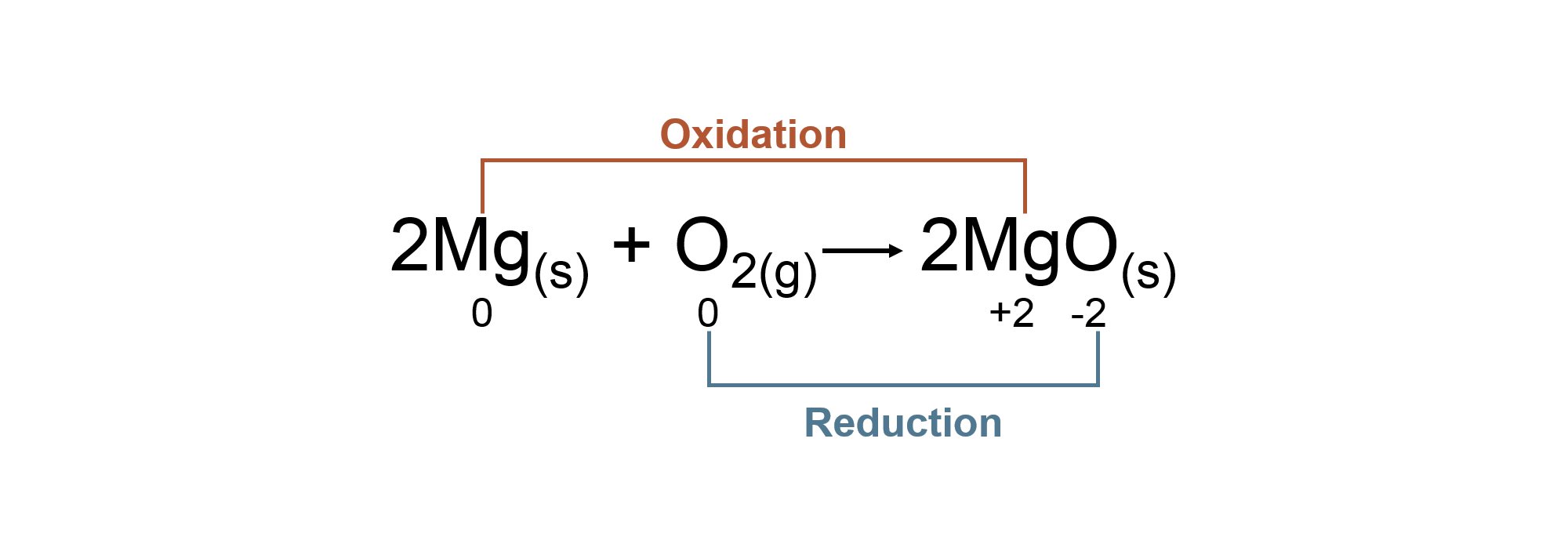
Balancing Chemical Equations:
Reaction equations must be balanced to account for all the products and reactants in the chemical reaction.

Balancing Chemical Reactions Guide:
1.
Write out the skeletal equation.
\(Fe_{(s)} + O_{2(g)} \to Fe_{2}O_{3(s)} \)
2.
Balance atoms that appear in more complex molecules first.
\(Fe_{(s)} + \)\(3O_{2(g)}\) \(\to\)\(2\)\(Fe_{2}\)
\(O_{3(s)}\)
Balance oxygen first.
3.
Balance Atoms that appear as free elements.
\(4Fe_{(s)} + \) \(3O_{2(g)} \to \)\(2Fe_{2}\)\(O_{3(s)}\)
Balance iron.
4.
Check:
Left Side
4 Iron
6 Oxygen
Right Side
4 Iron
6 Oxygen
Chemical Reactions Calculations:
We Know:
Molecular formulas describe the relative number of atoms in a compound.
Reaction equations use molecular formulas to describe equations.
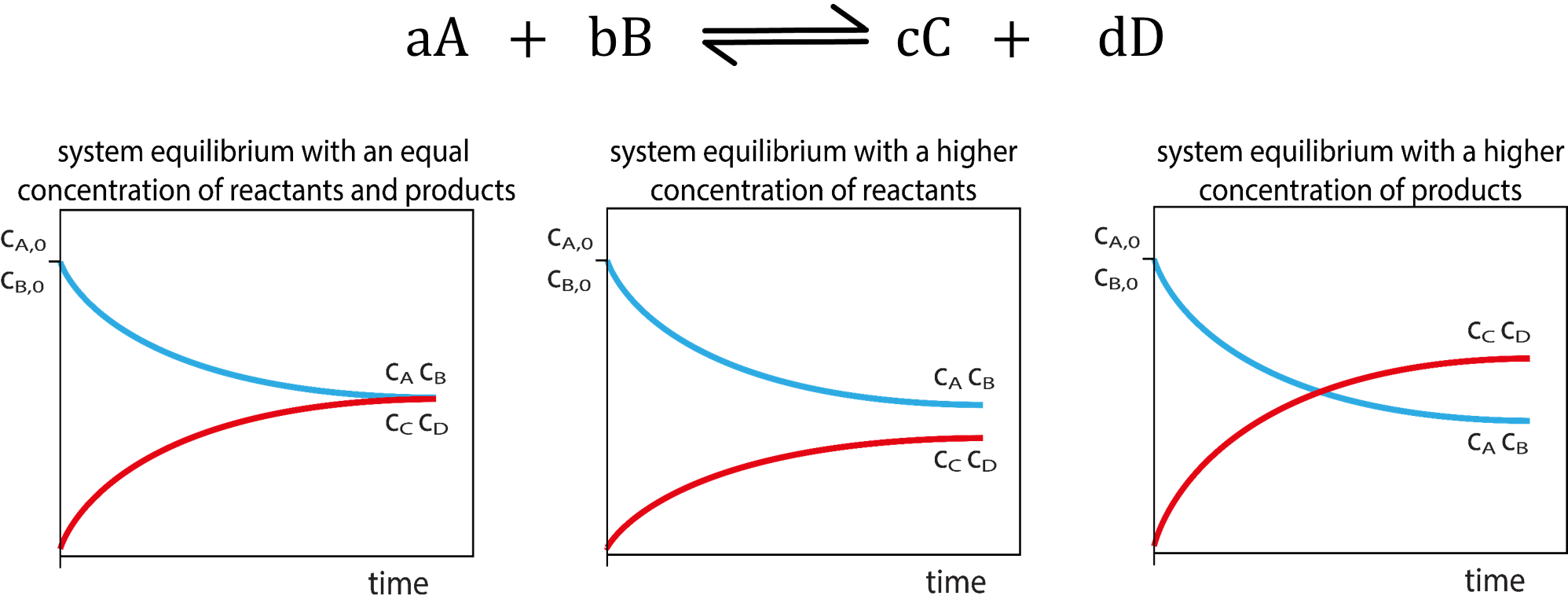
Stoichiometry:
A balanced chemical reaction equation can be used to calculate related reaction quantities!
ALWAYS balance reaction equations FIRST in all chemical reaction problems.
Stoichiometry: Using chemical equations to solve chemical reaction problems.
We Know:
In a chemical reaction reactants will rearrange in various ways to form products.
A balanced chemical reaction equation can be used to relate reactant and product quantities.
What if there is more of one reactant than another?
Limiting Reagent:
In a chemical reaction the reactant that has the smallest amount determines the amount of products that will be produced.
Limiting Reagent: The reactant that limits the amount of products that can be formed in a chemical reaction.
We Know:
Products are formed in a chemical reaction.
Yield:
The amount of products formed is referred to as a the ‘yield’ of the chemical reaction.
Click/tap to view definitions
Concentration:
The amount of dissolved solvent in a specific amount of solute.
Molarity:
Moles of solute per liter of solution.
\(M = \frac{\text{mol}}{L} = \text{mol}\)x\( L^{-1}\)
Concentration Formula:
\(M_{1}V_{1} = M_{2}V_{2}, M = \text{molarity} = \frac{\text{mol}}{L}\)
and V = volume = L
Many of the chemical reactions we are interested in occur in solution. Chemical problems and laboratories use solutions and solution concentrations frequently!